Abstract
CD147 is highly expressed in hepatocellular carcinoma (HCC) and associated with the invasion and metastasis of HCC. The efficacy of I131‐metuximab (I131‐mab), a newly developed agent that targets CD147, as a radio‐immunotherapy for local HCC, has been validated in clinical practice. However, the synergistic anticancer activity and molecular mechanism of different conjugated components within I131‐mab remain unclear. In this study, the cytological experiments proved that I131‐mab inhibited the proliferation and invasion of HCC cells. Mechanically, this inhibition effect was mainly mediated by the antibody component part of I131‐mab, which could reverse the epithelial–mesenchymal transition of HCC cells partially by suppressing the phosphorylation of VEGFR‐2. The inhibitory effect of I131 on HCC cell proliferation and invasion is limited, whereas, when combined with metuximab, I131 significantly enhanced the sensitivity of HCC cells to CD147‐mab and consequently reinforced the anticancer effects of CD147‐mab, suggesting that the two components of I131‐mab exerted synergistic anti‐HCC capability. Furthermore, the experiments using SMMC‐7721 human HCC xenografts in athymic nude mice showed that I131‐mab and CD147‐mab significantly inhibited the growth of xenograft tumors and that I131‐mab was more effective than CD147‐mab. In conclusion, our results elucidated the mechanism underlying the anti‐HCC effects of I131‐mab and provided a theoretical foundation for the clinical application of I131‐mab.
1 INTRODUCTION
Hepatocellular carcinoma (HCC) is a common malignant tumor that is commonly treated with surgery, minimally invasive treatment and transcatheter arterial chemoembolization (TACE) (Li & Yeo, 2017; Ramaswami et al., 2016). However, postoperative recurrence and metastasis remain important factors that affect the survival of patients with HCC (Grandhi et al., 2016; Yagci, Cetin, & Ercin, 2017). In particular, in HCC treated with chemotherapy or TACE, the residual cancer cells are found to be more invasive and metastatic (Su, 2016; Wan et al., 2016; Yu, Park, Park, & Yoon, 2016). Thus, further studying the genetic and biological properties of HCC is important to identify specific molecular markers of HCC recurrence and metastasis, develop new anti‐HCC agents and ultimately improve the quality of life of HCC patients.
Immunotherapy is an important approach of comprehensive cancer therapy. Specifically, HCC cells strongly express CD147 antigen, which is related to the invasion and metastasis of HCC (Chen et al., 2016). Thus, CD147, a highly glycosylated transmembrane protein and a member of the immunoglobulin superfamily, is an effective target for HCC immunotherapy. CD147 expression is up‐regulated in many tumors and is especially high in HCC tissue and HCC cell lines, where it is expressed in up to 75%. Conversely, it is not expressed in normal hepatic tissue and normal hepatic cell lines (Dai et al., 2009; Gou et al., 2009). Moreover, the over‐expression of CD147 can induce the expression and secretion of matrix metalloproteinase‐2 (MMP‐2) and matrix metalloproteinase‐9 (MMP‐9), which degrade extracellular matrix (ECM) and promote tumor invasion and metastasis by interfering with mesenchymal cells (Cui et al., 2012; Wang et al., 2010). To target CD147, a highly specific monoclonal antibody, HAB18 or metuximab, was developed and conjugated to the radioisotope I131. The resultant I131‐metuximab (I131‐mab) is a radio‐immunotherapy injection that was officially approved by the CFDA in 2007, being under the trade name Licartin for the treatment of local HCC (Ma & Wang, 2015; Wu, Shen, Xia, & Yang, 2016). I131‐mab exhibits several advantages, such as its specificity, rapid response and lethality to cancer cells. The preliminary results of a phase IV clinical trial of TACE combined with I131‐mab for the treatment of advanced HCC at eight Chinese institutions showed that I131‐mab is a safe and effective agent for the treatment of advanced HCC (Xu et al., 2007) and can effectively delay HCC relapse after hepatic transplantation (Wu et al., 2010, 2012). Moreover, the combination of TACE and I131‐mab can significantly prolong progression‐free survival and the overall survival of patients with advanced HCC according to the Barcelona clinical staging (BCLC) (Li et al., 2009). Among the 167 patients with HCC in this study, the 1‐year survival rate was significantly higher for patients receiving the combination treatment than that of patients only receiving the TACE treatment. The extrahepatic metastasis incidence in the combined treatment group was 2.94%, which was significantly lower than the 8.57% rate in the TACE group. This difference suggests that I131‐mab can reduce the extrahepatic metastasis incidence in patients after TACE treatment.
The molecular mechanisms underlying the acceleration effect of CD147 on HCC invasion and metastasis and the synergistic inhibition effect of I131‐mab on HCC progression have been reported (Bian et al., 2014; Gou et al., 2016). Specifically, CD147 can activate the transforming growth factor‐β (TGF‐β) signaling pathway to promote the expression and secretion of MMPs in tumor cells and mesenchymal cells, which induces the epithelial–mesenchymal transition (EMT) in cancer cells and contributes to tumor invasion and metastasis (Ru, Wu, Chen, & Bian, 2015; Xu et al., 2007). Growing evidence shows that EMT plays an important role in the molecular mechanisms underlying HCC occurrence and development. During the process of EMT, cells of epithelial origin transform into mesenchymal cells under specific physiological and pathological conditions, and this transition is accompanied by a series of phenotypic and behavioral changes within cells (Jayachandran, Dhungel, & Steel, 2016). EMT is not only involved in embryonic development but also plays an important role in the pathology of many diseases, such as fibrosis and cancer (Nieto, Huang, Jackson, & Thiery, 2016). In cancer cells, EMT can decrease cell adhesion and increase cell motility, and these changes promote invasion, metastasis and the formation of a new tumor (Giannelli, Koudelkova, Dituri, & Mikulits, 2016; Polyak & Weinberg, 2009). I131‐mab specifically binds to CD147, which is located on the surface of HCC cells, to inhibit TGF‐β signaling and MMP secretion. This inhibition consequently interrupts EMT process and suppresses the invasion and metastasis of cancer cells.
The efficacy of TACE in combination with I131‐mab has been confirmed, and the mechanisms underlying this effect have also been partially elucidated. However, multiple factors are involved in these mechanisms. Do the mechanisms interact? Is the effect synergistic? In addition to the I131‐mab‐mediated inhibition of TGF‐β signaling to interrupt EMT, are other signaling pathways involved in this process? Furthermore, studies are needed to answer these questions. The work described herein shows that I131‐mab can reverse the EMT of HCC cells, and metuximab, a component of I131‐mab, plays an important role in the reversion effect. I131 can increase sensitivity of HCC cells to metuximab to synergistically enhance the efficacy of treatment. Moreover, the mechanism by which I131‐mab reverses the EMT of HCC cells is related to VEGFR‐2 signaling. Overall, this work showed that I131‐mab may interrupt EMT of HCC by inhibiting VEGFR‐2 signaling to prevent HCC metastasis.
2 RESULTS
2.1 Inhibition of HCC cell proliferation by I131‐mab
Western blotting was used to measure the expression levels of CD147 in a variety of cell lines, and CD147 was expressed in HepG2, Hep3B, SMMC‐7721, MHCC97H and MHCC97L cells, with HepG2, Hep3B and MHCC97H cells expressing higher levels of CD147. MHCC97L cells expressed the lowest level of CD147, and WRL‐68 cells were negative for the expression of CD147 (Figure 1a). Based on these results, we selected CD147‐negative WRL‐68 cells to investigate the cytotoxicity of I131‐mab and CD147 antibody (CD147‐mab). CD147‐mab did not affect cell viability, whereas I131‐mab exerted a dose‐dependent effect on cell viability. The IC20 and IC50 values of I131‐mab on WRL‐68 cells were 8.09 μCi/100 μl and 12.69 μCi/100 μl, respectively (Figure 1b). Because WRL‐68 cells did not express CD147 and were not inhibited by CD147‐mab, the cytotoxicity of I131‐mab was attributed to the conjugated I131. Because the unit of measurement of I131‐mab and I131 was μCi and the unit of measurement of CD147‐mab is μM, therefore, to establish the concentration equivalence relation between I131‐mab and CD147‐mab, we regarded that the concentrations of I131‐mab corresponding to IC20 and IC50 could be served as the reference concentrations of I131 and consequently calculated the molar concentrations of CD147‐mab to be 5.62 μM/100 μl and 8.82 μM/100 μl, respectively.

The tested cells were treated with I131‐mab, I131 (both agents administered at doses of 8.09 μCi/100 μl or 12.69 μCi/100 μl) and CD147‐mab (5.62 μM/100 μl and 8.82 μM/100 μl corresponding to the concentration of I131‐mab) to examine their effects on the proliferation of HCC cells. The results showed that I131‐mab was significantly more cytotoxic to all HCC cell lines than CD147‐mab or I131 alone, and specifically, viability was lowest in HepG2, Hep3B and MHCC97H cells, and doses of 8.09 μCi/100 μl and 12.69 μCi/100 μl resulted in viabilities lower than 30% and 10%, respectively. In contrast, I131‐mab was weakly cytotoxic to MHCC97L, resulting in viabilities exceeding 50% at doses of 8.09 μCi/100 μl and 12.69 μCi/100 μl. Moreover, CD147‐mab was significantly more cytotoxic to HepG2, Hep3B, SMMC‐7721 and MHCC97H cells than I131, whereas the effects of I131 and CD147‐mab on MHCC97L cells did not significantly differ (Figure 1c).
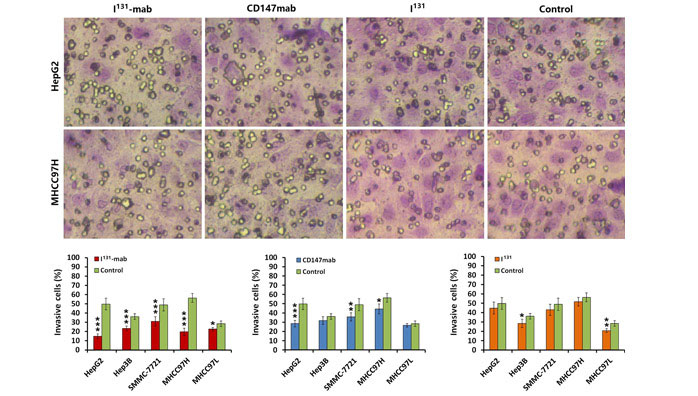
2.2 Inhibition of HCC cell invasion by I131‐mab
To verify the effect of I131‐mab on HCC cell invasion, a Transwell device was used to assess cell invasion. Both I131‐mab and CD147‐mab significantly inhibited the invasion of the HCC cell lines. Specifically, I131‐mab inhibited HepG2, Hep3B, SMMC‐7721, MHCC97H and MHCC97L cell invasion more effectively than CD147‐mab, whereas the effects of CD147‐mab on the invasion of Hep3B and MHCC97L did not significantly differ from the control group. These results suggested that inhibition of HCC cells by I131‐mab or CD147‐mab was closely associated with CD147 expression. I131 alone exerted a limited effect on HCC cell invasion, and this effect only differed between the treated and control groups for MHCC97L cells (Figure 2).

2.3 I131‐mab reversed HCC cell EMT via VEGFR‐2 signaling
To elucidate the mechanism by which I131‐mab inhibits HCC cells, we compared some markers related to EMT in HCC cells treated with I131‐mab, I131 and CD147‐mab. E‐cadherin expression was significantly increased, whereas the expression levels of N‐cadherin and vimentin were decreased in all HCC cells treated with I131‐mab and CD147‐mab. Conversely, I131 did not affect the expression levels of these proteins (Figure 3a). These results suggested that I131‐mab reversed HCC cell EMT via the CD147 antibody in the conjugated molecules but not via I131.

VEGFR expression was further studied in HCC cells treated with I131‐mab, CD147‐mab and I131. The results showed that VEGFR‐2 phosphorylation (p‐VEGFR‐2) significantly decreased in HCC cells treated with I131‐mab and CD147‐mab, whereas the levels of VEGFR‐1, p‐VEGFR‐1 and VEGFR‐2 did not change. I131 alone did not affect the expression levels of these proteins in HCC cells (Figure 3b). These results suggest that I131‐mab reversed EMT by inhibiting the phosphorylation of VEGFR‐2 in HCC cells.
2.4 I131 increases the sensitivity of HCC cells to the cytotoxicity of CD147‐mab
By MTT assay, we found that I131 did not inhibit the proliferation of all HCC cells at doses <8.09 μCi/100 μl, whereas the cytotoxicity of 5.62 μM/100 μl CD147‐mab (concentration corresponding to the I131 dose) varied among HCC cell lines. We cotreated cells with I131 (8.09 μCi/100 μl) and CD147‐mab (5.62 μM/100 μl) to compared the effect of this treatment to that of CD147‐mab alone. We found that the viability of all HCC cell lines significantly decreased in response to the combined treatment (Figure 4a). The results of cell invasion capability were coincident with the MTT assay (Figure 4b,c). The results suggested that I131 increases the sensitivity of HCC cells to CD147‐mab and consequently enhances the cytotoxicity of this agent.

2.5 Inhibition of SMMC‐7721 human hepatoma xenografts by I131‐mab
The inhibition of HCC by I131‐mab and the molecular mechanisms underlying this inhibition were verified in nude mice harboring SMMC‐7721 human hepatoma xenografts. Treating these xenografts with I131‐mab injection markedly inhibited tumor growth speed; specifically, the tumor volume was significantly smaller in the I131‐mab treatment group than that in the control group 7 days after the first injection, and this difference increased over time. The efficacy of CD147‐mab group was evident 14 days after the start of treatment, whereas no noticeable effects were observed in the I131 treatment group, even at the end of the observation period (Figure 5a). After 21 days of treatment, the tumor volume in the control group exceeded the standard limit, and the observation was ended. The tumors were dissected and weighed, and the tumor weight was lowest in the I131‐mab group, followed by the CD147‐mab group. There is no difference in tumor weight between the I131 group and the control group (Figure 5b). Tissue sections were obtained from the hepatoma xenografts, and immunohistochemistry staining was used to measure E‐cadherin and p‐VEGFR‐2 expression. The results showed that E‐cadherin expression was up‐regulated, whereas p‐VEGFR‐2 expression was down‐regulated in the I131‐mab and CD147‐mab groups (Figure 5c).

3 DISCUSSION
EMT has been observed in many human tumors, and extracellular signaling molecules that induce EMT in tumor cells include MMP‐2, MMP‐3, MMP‐9, type I or III collagen, hepatocyte growth factor (HGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), TGF‐β and tumor necrosis factor‐α (TNF‐α). EMT also modulates the activity of multiple signaling pathways, such as TGF‐β, Wnt, PDGF, Notch, Hedgehog, Akt, PI3K, NF‐κB and Ras, via the Snail family of zinc finger transcription factors (Snail1, Snail2 and Snail3) and the helix‐loop‐helix structure of transcription factors (Twist, ZEB 1 and ZEB2/SIP1) to down‐regulate the epithelial markers E‐cadherin and keratin or up‐regulate the mesenchymal markers N‐cadherin and vimentin (Hanna & Shevde, 2016; Lee & Kong, 2016; Moustakas & Heldin, 2016; Xu, Yang, & Lu, 2015; Zhang, Tian, & Xing, 2016). EMT increases the malignancy of cancer cells because cells lose apical–basal polarity and cell junctions and acquire a migratory mesenchymal phenotype. These cells then invade the lymphatic or vascular system and spread to different sites or organs to grow and form metastatic tumors (Jayachandran et al., 2016; Kölbl, Jeschke, & Andergassen, 2016).
TACE is an important treatment for HCC but has been clinically associated with increases in distant metastasis due to residual cancer cells in many patients with HCC (Xue et al., 2016). This phenomenon has greatly compromised the long‐term efficacy of TACE. Moreover, TACE can induce tissue hypoxia, which down‐regulates E‐cadherin and up‐regulates of N‐cadherin in residual cancer cells. Thus, hypoxia may mediate the negative effects of TACE by inducing EMT in cancer cells (Fransvea, Angelotti, Antonaci, & Giannelli, 2008; Xue et al., 2015). EMT enhances the invasion and metastasis of cancer cells, reduces the sensitivity or increases the resistance of HCC to chemotherapeutic agents (Bae et al., 2014; Nishida, Kitano, Sakurai, & Kudo, 2015) and contributes to the immune escape of HCC cells (Chockley & Keshamouni, 2016; Ye et al., 2016).
The studies of the relationship between EMT and the metastasis of HCC have provided an effective target for HCC treatment. LY2109761, a TGF‐β 1 receptor kinase inhibitor, inhibits EMT to reduce vascular invasion and metastasis in HCC cells (Baldassarre et al., 2012). Blocking the effects of upstream loop regulatory factors of EMT, such as inhibition of NF‐κB/miR‐448, can improve the response of cancer cells to chemotherapeutic agents (Li et al., 2011). CD147, under the regulation of slug at transcriptional level, can promote EMT process in HCC via TGF‐β signaling (Wu et al., 2011). Furthermore, hypoxia caused by TACE can up‐regulate CD147 expression in HCC cells (Gou et al., 2016), and the over‐expression of CD147 enhances EMT in cancer cells by activating TGF‐β signaling. Thus, I131‐mab, which targets CD147, may specifically inhibit EMT to attenuate tumor development and metastasis. In nude mouse HCC models, I131‐mab effectively inhibited the growth and metastasis of HCC and inhibited MMPs and VEGF expression in the para‐tumor microenvironment (Wu et al., 2011). We conducted a prospective controlled clinical study in which the combination of TACE and I131‐mab was used to treat intermediate‐stage HCC (BCLC staging). Specifically, 68 patients with intermediate‐stage HCC were included in the combined treatment group, and 70 patients were included in the TACE alone group. The median survival time was 26.7 months in the combined treatment group but only 20.6 months in the control group, and the overall survival in the combined treatment group was significantly better than that in the control group (p = .038). The median time to progression was 18.6 months in the combined treatment group and superior to only 12.5 months in the control group (p = .046). In particular, the extrahepatic metastasis rate was 2.94% in the combined treatment group and 8.57% in the TACE group. These results suggested that I131‐mab reduces the risk of extrahepatic metastasis after TACE treatment (Wu et al., 2012).
In this work, the cytological experiments proved that I131‐mab inhibits the proliferation and invasion of HCC cells, and this inhibition is closely related to the level of CD147 expression. HCC cells expressing high levels of CD147 are more sensitive to I131‐mab. The inhibition effect of I131‐mab on HCC cells was mostly attributed to the CD147 antibody within the conjugated molecule. Although the inhibition of HCC cell proliferation and invasion by I131 alone is limited, when combined with CD147‐mab, I131 significantly enhanced the sensitivity of cancer cells to CD147‐mab and consequently enhanced the cytotoxicity of anticancer antibodies. This finding suggested that the two components of I131‐mab synergistically inhibited HCC. Both I131‐mab and CD147‐mab can reverse the EMT of HCC cells partially by inhibiting the phosphorylation of VEGFR‐2 and then reduce the capabilities of proliferation and metastasis of HCC cells. The experiments using SMMC‐7721 human hepatoma xenografts in athymic nude mice also proved that I131‐mab and CD147‐mab significantly inhibited xenograft tumors, and I131‐mab was more effective than CD147‐mab in this inhibition. The synergistic effect of conjugated I131‐mab was attributed to I131 because I131 alone did not significantly inhibit the proliferation and invasion of HCC. Our results elucidated the mechanism underlying the antiproliferative and antimetastatic effects of I131‐mab and provided a theoretical foundation for the clinical application of I131‐mab.
4 EXPERIMENTAL PROCEDURES
4.1 Cells and reagents
Human hepatoma cell lines (HepG2, Hep3B, SMMC‐7721, MHCC97H and MHCC97L) and normal hepatic cells (WRL‐68) were provided by Cell Bank, Institute of Cell Biochemistry, Chinese Academy of Sciences Shanghai Institutes for Biological Sciences (Shanghai, China). The cells were cultured according to the manufacturer's protocol. I131‐mab was developed by the Chengdu Huasun Group Inc., Ltd. (Chengdu, China). CD147‐mab and Sodium Iodide [131] Capsules (I131) were purchased from Abcam Trading Company Ltd. (Shanghai, China) and Atom‐Hitech Co., Ltd. (Beijing, China), respectively.
4.2 Cell proliferation assay
A tetrazolium colorimetric assay (MTT assay) was used to assess the effects of I131‐mab, CD147‐mab and I131 on the proliferation of HCC and normal cells. Briefly, cells were harvested during the logarithmic growth phase and seeded in 96‐well plates at 1 × 104 cells/100 μl/well, and then cultured for 24 hr; serum‐free culture medium was used to dilute I131‐mab, and cells were treated with various concentrations of this agent, with eight duplicates per concentration. After incubated for 2 hr, the medium was then replaced with medium containing 10% serum (100 μl/well). After 48 hr of culture, the medium was replaced with 0.1 M PBS solution (100 μl/well), and MTT labeling reagent (10 μl/well for a final concentration of 0.5 mg/ml; Roche Diagnostics GmbH, Shanghai, China) was then added. After 4 hr of incubation, the solubilization solution (10% SDS in 0.01 mol/L HCl, 100 μl/well) was added for overnight, the absorbance was measured at 490 nm, and the values were plotted to assess cell viability and calculate the IC20 and IC50 of each concentration of I131‐mab and its controls.
4.3 Cell invasion assay
The upper Transwell chambers (8 μm pore size, Corning, Tewksbury, USA) were coated with 50 μl of 1:6 diluted Matrigel (BD Biosciences, San Jose, USA), and the lower chambers were filled with 500 μl of 10% fetal bovine serum. The Transwell chambers were placed in a well of a 24‐well plate. Harvested cells were seeded into the upper Transwell chamber at 1 × 105 cells/well and cultured for 24 hr; I131‐mab was diluted in serum‐free culture medium and added to the wells for a variety of final concentrations. The cells were incubated for 2 hr, and the medium was replaced with medium containing 10% serum (100 μl/well) after 48 hr of incubation. The Transwell inserts were removed, and the cells were stained with 0.1% crystal violet for 20 min before being counted under a microscope; three random fields were photographed (200× magnification).
4.4 Immunoblotting
The above‐studied cells were seeded in a 24‐well plate at 1 × 105 cells/well and cultured for 24 hr. I131‐mab was diluted in serum‐free culture medium and added to the culture wells at various concentrations (based on the protocol). These cells were cultured for 2 hr. Medium containing 10% serum (100 μl/well) was used to replace the previous medium. After 48 hr of incubation, the cells were harvested. Western blotting was used to measure the expression of the target proteins. The antibodies used in the experiments are listed below: rabbit anti‐CD147 (Abcam Trading Company Ltd., Shanghai, China); mouse anti‐E‐cadherin, mouse anti‐N‐cadherin, mouse antivimentin, rabbit anti‐VEGF‐C antibodies (Cell Signaling Technology, Danvers, MA, USA); and mouse anti‐VEGFR‐1, mouse anti‐VEGFR‐2, rabbit anti‐phospho‐VEGFR‐1 and rabbit anti‐p‐VEGFR‐2 (Cell Applications Inc., CA, USA).
4.5 Human hepatoma xenografts in nude mice
A total of 25 healthy, purebred BALB/C male mice aged 4 weeks were purchased from the SLAC Experimental Animal Centre of the Chinese Academy of Sciences (Shanghai, China). SMMC‐7721 cells were harvested during the logarithmic growth phase, and cell suspension was prepared. The cells (5 × 105 cells in 100 μl per mouse) were subcutaneously injected into the right flanks of nude mice, and tumors formed 12 days after inoculation. The three mice with the largest tumors and the two mice with the smallest tumors were excluded. The remaining 20 mice were randomly divided into four groups (the I131‐mab group, the CD147‐mab group, the I131 group and the control group). Animals in the I131 and I131‐mab groups received injections of I131 or I131‐mab at multiple sites (dose: 12.69 μCi/100 μl per mouse); animals in the CD147‐mab group received CD147‐mab at a dose of 8.82 μM/100 μl per mouse. The animals were injected every other day for a total of five injections. The control mice were injected with saline (100 μl per mouse per injection). After treatment, tumor size was measured weekly, and the following formula was used to calculate tumor volume: “maximum diameter × minimum diameter2 × 0.5.” The experiment was terminated immediately when the mean tumor volume exceed 2,000 mm3 in any group, as defined by the Animal Ethics Committee of the Second Military Medical University. At the end of observation, the mice were killed with over‐doses anesthesia. The tumor specimens were harvested and weighed, and the tumor tissue was fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned and immunohistochemically stained.
4.6 Immunohistochemistry
The sections of paraffin‐embedded SMMC‐7721 hepatoma xenografts were subjected to streptavidin–peroxidase immunohistochemistry to detect the expression of E‐cadherin and p‐VEGFR‐2. The antibodies used for staining were the same as those used for Western blotting. The percentages of positive cells in all sections were determined by counting the cells in five high‐magnification fields.
4.7 Statistical analysis
The cytological experiments were repeated three times independently, and a total of five animals were included in each group. The data are expressed as the mean ± SD. An analysis of variance (ANOVA) and a t test were used for the statistical analysis with the PASW Statistics 18 software. p value less than .05 was considered significant.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81301302; 81773251; 81702735), and the Science and Technology Commission of Shanghai Municipality (15411966100).
AUTHOR CONTRIBUTIONS
L.W., B.S., X.L. and C.L. conceived the experiments. H.Q., L.C. and C.S. conducted the experiments. L.W., B.S., X.L., Y.Y. and F.S. analyzed the results. All authors reviewed the manuscript.
The information comes from:
https://onlinelibrary.wiley.com/doi/full/10.1111/gtc.12545