Production of 177Lu for Targeted Radionuclide Therapy: Available Options
Abstract
Background: This review provides a comprehensive summary of the production of 177Lu to meet expected future research and clinical demands. Availability of options represents the cornerstone for sustainable growth for the routine production of adequate activity levels of 177Lu having the required quality for preparation of a variety of 177Lu-labeled radiopharmaceuticals. The tremendous prospects associated with production of 177Lu for use in targeted radionuclide therapy (TRT) dictate that a holistic consideration should evaluate all governing factors that determine its success. Methods: While both “direct” and “indirect” reactor production routes offer the possibility for sustainable 177Lu availability, there are several issues and challenges that must be considered to realize the full potential of these production strategies. Results: This article presents a mini review on the latest developments, current status, key challenges and possibilities for the near future. Conclusion: A broad understanding and discussion of the issues associated with 177Lu production and processing approaches would not only ensure sustained growth and future expansion for the availability and use of 177Lu-labeled radiopharmaceuticals, but also help future developments.
Introduction
Over the last several years, the 177Lu radionuclide has attracted considerable attention and exhibited great promise in the research, commercial and clinical communities for use in a variety of therapeutic procedures [1–8]. Despite being a late entrant, 177Lu has not only consolidated its potential, but also established a strong foothold at the forefront of TRT. In a relatively short time span, 177Lu has virtually pervaded all areas of in vivo radionuclide therapy and may be poised to become a key therapeutic radionuclide of choice for TRT. The growing interest in the use of 177Lu in targeted molecular therapies has primarily developed from recent unprecedented advances in molecular and cell biology, which include the use of peptides targeted to cell surface receptors, which are overexpressed on the surface of tumor cells.
Lutetium-177 decays in 76 % of events (E β(max) =0.497 MeV) to the stable ground state of 177Hf with a half-life of 6.65 days and decays in 9.7 % of events (E β(max) =0.384 MeV) and 12 % of the time (E β(max) = 0.176 MeV) to an excited state of 177Hf that lies 0.24967 MeV and 0.32132 MeV above the ground state, which de-excites to the ground state with the photon emission. During these radioactive decay events, 177Lu emits β- particles with an E β(max) of 497 keV (78.6 %), 384 keV (9.1 %) and 176 keV (12.2 %) and low-energy gamma photons [Eγ = 113 keV (6.6 %), 208 keV (11 %)] [9, 10]. A simplified decay scheme for 177Lu is shown in Fig. 1.
Increasing use of 177Lu in nuclear medicine procedures has been impressive, and widespread applications of 177Lu therapeutic agents have not only stimulated progress of TRT, but have also been responsible for stimulating the growth of these therapeutic methods. The utility of 177Lu is thus continually evolving, well entrenched in the arena of targeted radionuclide therapy, and has unveiled a broad spectrum of 177Lu-labeled therapeutic radiopharmaceuticals for treating a wide range of diseases. It is surmised by many that diffusion of 177Lu into nuclear medicine has not only brought spectacular developments in radionuclide therapy, but has also prompted a perceptible shift of the radiotherapeutic method toward the treatment of some diseases. The remarkable prospects and impetus associated with the use of 177Lu-labeled radiopharmaceuticals in TRT have been the major factors evoking excitement among researchers and capturing the imagination of the clinical community thanks to advances in molecular and cellular biology.
While myriad factors contribute to the success of 177Lu-labeled radiopharmaceuticals, the cost-effective availability of sufficient activity levels of 177Lu that have the required quality is a key determinant underpinning the success of using 177Lu in in vivo targeted therapy. The recent surge of interest in the use of 177Lu in TRT has been the motivation to provide this review on the production and processing of this emerging radionuclide. This article focuses on a discussion of the 177Lu production strategies that are currently used and other approaches that may have considerable potential in the foreseeable future.
Why is 177Lu Expected to Be So Important in Targeted Radionuclide Therapy?
The striking diffusion and exciting perspectives of 177Lu in TRT are primarily attributed to the following.
The mean penetration range of β− particles emitted by 177Lu in soft tissue is 670 μm, making this radionuclide ideal for delivering energy to small volumes, including micrometastatic disease, and tumor cells near the surface of cavities. Lutetium-177 is found to be effective in localizing cytotoxic radiation in relatively small areas and proficient in destroying small tumors as well as metastatic lesions (typically less than 3 mm diameter) with less damage to surrounding normal tissue.
The emission of low-energy gamma photons enables imaging the biodistribution and excretion kinetics with the same radiolabeled preparation used for therapy and allows dosimetry to be performed before and during treatment as well. This property is important for “personalized” medicine for the development of “theranostic” agents for combined diagnostic and therapeutic use that can deliver therapy to individual cells in affected tissues.
The emission of moderate-energy beta β- particles as well as low-energy gamma photons results in a relatively low radiation dose and therefore offers the potential to handle relatively high 177Lu activity levels during radiopharmaceutical preparation and formulation of radiopharmaceuticals as well as during patient administration.
Lutetium exclusively exists in the +3 oxidation state, which precludes any solution chemistry reduction-oxidation complications and commonly forms nine coordination complexes. This property therefore provides the potential for radiolabeling a variety of molecular carriers, which include small molecules, and peptides, proteins and antibodies with the specific desired characteristics for therapy. The chemical characteristics of Lu+3 are suitable for peptide and protein radiolabeling by attachment of a bifunctional chelating agent (BFCA) through a metabolically resistant covalent bond.
The 6.65-day half-life of 177Lu offers extended time periods, which may be required for the use of more sophisticated procedures to radiolabel and purify 177Lu-labeled radiopharmaceuticals, and for performing quality control and administration. The use of a longer lived therapeutic radionuclide such as 177Lu is particularly well suited for the radiolabeling of antibodies that have slow targeting kinetics.
The relatively long 6.65-day physical half-life of 177Lu not only minimizes decay loss, which may be encountered during the transportation and distribution to users, but also provides excellent logistical advantages for shipment to sites distant from the reactor production facility as well as radionuclide-processing facilities.
A wide range of 177Lu radiopharmaceuticals has been successfully developed and evaluated. The in vivo applications of key 177Lu radiopharmaceuticals for a variety of therapeutic procedures include peptide receptor radionuclide therapy [11–26], bone pain palliation [27–33], radiation synovectomy [34–39] and radioimmonutherapy [40–46]. There is a steadily expanding list of 177Lu-labeled radiopharmaceuticals that is currently being evaluated at the preclinical research or at product development stages; these may potentially be used in vivo in humans for evaluation for radionuclide therapy [1–3]. A summary of key 177Lu-labeled radiopharmaceuticals currently used in TRT is depicted in Table 1.
Table 1
Key examples of current 177Lu-labeled radiopharmaceuticals
| Application | Chemical form | Usual administered activity | References |
|---|---|---|---|
| Peptide receptorradionuclide therapy (PRRT) | [DOTA0,Tyr3]octreotate DOTA-TATE,DOTA-TOC, DOTA-NOC | 7.4 GBq (200 mCi) per course. It is bound to 180 to 300 μg of the peptide with the chelator DOTA, [DOTA0,Tyr3] octreotate | [11–26] |
| Bone pain palliation in skeletal metastases | EDTMP DOTMP | 1.2 to 2.6 GBq | [27–33] |
| Radiation synovectomy | Hydroxyapatite (HA) | ~400 ± 30 GBq (10.8 ± 0.8 mCi) | [38] |
| Radioimmonutherapy | Monoclonal antibodies (J591, cG250, J591) | Not established | [2] |
Production of 177Lu
The opportunity for production of 177Lu in research reactors throughout the world indicates that all pertinent factors should be evaluated and assessed. Essentially every conceivable production and processing strategy has been exploited with a view to obtain 177Lu in a chemical form having acceptable radionuclidic and radiochemical purity. Since the inherent success of any production strategy requires a thorough knowledge of all the pertinent key factors, the issues underlying the utility of 177Lu in TRT are discussed below.
Specific Activity of 177Lu
Before discussing production of 177Lu in detail, it is relevant to discuss the importance of the specific activity (SA) of 177Lu, since this key factor dictates its utility for TRT.
No-Carrier-Added 177Lu
Radionuclides have maximum theoretical specific activity values referred to as “carrier-free” when all the atoms contain one isotope of the element. Carrier free (CF) thus denotes a radionuclide having 100 % isotopic abundance, i.e., free from any stable isotopes. A radionuclide is characterized as no carrier added (NCA or n.c.a.) to which no carrier atoms have been added and for which precautions have been taken to minimize contamination with stable isotopes of the element in question. It does not necessarily mean, however, 100 % isotopic abundance. ‘Carrier free’ is an idealistic situation and hence the current term used is ‘no carrier added (NCA)’ for a preparation having a specific activity value that approaches the calculated maximum theoretical specific activity.
The theoretical specific activity of ‘carrier-free (CF)’ 177Lu is calculated from the following equation:
Here,
N →, number of 177Lu atoms.
λ →, decay constant of 177Lu.
T½ →, half life of 177Lu.
Neutron-capture characteristics, target impurities, secondary nuclear reactions, target “burn-up” and post-irradiation processing/cooling periods are the main parameters affecting the SA of the 177Lu product.
The Importance of 177Lu-Specific Activity
Conscientious harnessing of the nuclear and chemical characteristics of 177Lu in conjunction with the advancement in molecular and cellular biology has not only stimulated the progress of radionuclide therapy, but has also driven the field. As discussed earlier, the utility of 177Lu in radionuclide therapy has undergone rapid and continual evolutionary cycles. Lutetium-177-labeled therapeutic radiopharmaceuticals comprising small molecules, large biomolecules and particles are currently being evaluated for myriad of clinical applications [11–46].
While targeted radionuclide therapy is primarily based on selecting appropriate radiopharmaceuticals and targeting mechanisms, the number of target sites (receptors, cells, etc.) available for radiopharmaceutical targeting dictates the SA of 177Lu that is appropriate for a particular application. For instance, targeting to trabecular bone is considered a large-capacity site and does not require 177Lu with highly specific activity. For this reason, the 177Lu SA is basically of minimal consequence in applications involving preparation of 177Lu-labeled radiopharmaceuticals used for treatment of bone pain palliation, hepatocellular carcinoma (HCC) and synovectomy, since high masses of the low SA agents are administered. Under this premise, medium-low SA 177Lu produced by the “direct” route described below can generally be used. However, high SA 177Lu is required for other applications involving low capacity sites, which are present in low numbers, such as receptor sites for peptide and antibody therapy. In the field of peptide receptor radionuclide therapy (PRRT) with radiolabeled peptide analogs, use of high SA 177Lu constitutes a necessity owing to the limited concentrations of the different cellular cognate receptors expressed on the tumor cell surface. Similar is the case with radioimmunotherapy (RIT), where the use of radiolabeled monoclonal antibodies targets different tumor-associated antigens and exceeding the mass threshold for pharmacologic activity of the antibody could cause unwanted reactions. For this application, the tumor cell antigen concentrations are overexpressed compared to limited concentrations associated with normal cells. Unlike peptides, monoclonal antibodies are macromolecules with molecular weights of about 150,000 Da, so the specific activity values that can be achieved by adding one or two 177Lu atoms to each molecule will also be low. Hence, RIT also requires high-specific-activity radionuclide preparations.
177Lu Production Process
Both the “direct” and “indirect” reactor production routes can be followed to obtain 177Lu for nuclear medicine applications: The direct production route is based on neutron irradiation of 176Lu targets by the 176Lu(n,γ)177Lu reaction. The indirect 176Yb(n,γ)177Yb → 177Lu production route necessitates a chemical separation of 177Lu from the target 176Yb target atoms.
The direct and indirect reactor routes for production of 177Lu are shown in Fig. 2. Each route has specific advantages and disadvantages, which are elaborated in the following sections. The neutron activation products of natural lutetium and ytterbium targets along with the nuclear decay characteristics of the product radionuclides are given in Table 2.
Table 2
Neutron activation products of natural lutetium and ytterbium targets along with the nuclear decay characteristics of the product radionuclides
| Element | Target isotope | % Natural Abundance | Cross section σ (barn) | Activation product | Decay Mode | T1/2 | Decay product |
|---|---|---|---|---|---|---|---|
| Lu | 175Lu | 97.41 | 16.7 | 176mLu | β-, γ | 3.66 h | 176Hf |
| 6.6 | 176Lu | β-, γ | 4 × 1010 y | 176Hf | |||
| 176Lu | 2.59 | 2.8 | 177mLu | β-, γ & IT | 160.4 d | 177Hf (78.6 %) 177Lu (21.4 %) | |
| 2090 | 177Lu | β-, γ | 6.65 d | 177Hf | |||
| Yb | 168Yb | 0.13 | 2300 | 169Yb | EC | 32.02 d | 169Tm |
| 170Yb | 3.04 | 9.9 | 171Yb | Stable | |||
| 171Yb | 14.28 | 58.3 | 172Yb | Stable | |||
| 172Yb | 21.83 | 1.3 | 173Yb | Stable | |||
| 173Yb | 16.13 | 15.5 | 174Yb | Stable | |||
| 174Yb | 31.83 | 63 | 175Yb | β-, γ | 4.18 d | 175Lu | |
| 176Yb | 12.76 | 2.85 | 177Yb | β-, γ | 1.9 h | 177Lu |
Direct Production Route [176Lu(n,γ)177Lu]
The direct production route offers the following advantages.
The least intricate approach to target irradiation in a reactor and requires minor design changes in reactor irradiation and processing facilities.
Offers the potential to use the 176Lu2O3 target, which remains stable under irradiation conditions and is compatible with reactor irradiation.
Irradiated target processing is easy, fast and technically less demanding as simple target dissolution in dilute mineral acid on gentle warming suffices. The facility required for target processing is straightforward to install and maintain.
Has the flexibility to scale the increase or decrease levels of production in response to requirements by adjusting the target size.
Processing generates negligible levels of radioactive waste.
This production method represents the most inexpensive option to obtain 177Lu of requisite purity.
Unlike other medically useful radionuclides, the direct (n,γ) production route often offers the prospect of producing 177Lu with SA adequate for preparing receptor-specific therapeutic radiopharmaceuticals. This is possible because 176Lu has a very high thermal neutron capture cross section (σ = 2090 b, I0 = 1087 b) for formation of 177Lu. The neutron capture cross section of 176Lu does not follow the 1/v law, and there is a strong resonance very close to the thermal region [50, 54].
In spite of the advantages of the direct production route, some concerns that have been raised on the use of this production route include:
With a view to augmenting the 177Lu production yield as well as specific activity, the possibility of using enriched 176Lu targets is necessary owing to the limited natural abundance (2.6 %) of 176Lu in the unenriched target.
The specific activity of 177Lu obtained by this method is generally 740–1,110 GBq (20–30 Ci)/mg versus the theoretical SA value of 4.07 TBq (110 Ci)/mg. This indicates that only 25 % of the atoms are 177Lu, and 75 % consisting of the product mixture are nonradioactive contaminating 175/176Lu atoms. Thus, the maximum obtainable SA that can be achieved only with high-flux reactors is about 70 % of the theoretical value.
These SA values are adequate for preparation of the 177Lu-labled agents used for bone pain palliation, synovectomy, treatment of liver cancer and some other applications.
However, while the directly produced 177Lu 740–1,110 GBq (20–30 Ci)/mg can be sufficient for PRRT, the SA of course decreases with time; therefore, the shelf life of 177Lu obtained by this method is limited for PRRT and for use in other applications that may require higher SA.
A unique feature of this method is the co-production of 177mLu, the presence of which can be associated with radiation protection and waste disposal challenges in some countries, which are discussed in more detail later in this section.
Target Selection
175Lu (97.4 %) and 176Lu (2.6 %) are the two naturally occurring isotopes of lutetium. While only 175Lu is truly “stable,” 176Lu decays by beta decay with a half-life of 4 × 1010 years. While undertaking the production of 177Lu, the Lu2O3 is the preferred chemical form because of its chemical and thermal stability during irradiation and its solubility in dilute mineral acid. There appears to be great interest in the use of an enriched 176Lu target in view of the explicit need to obtain high-specific-activity 177Lu amenable for radionuclide therapy. Additionally, the targets used for production should be of exceptionally high purity as isotopic impurities are likely to decrease the specific radioactivity of the produced 177Lu owing to high target nuclide burn-up during high neutron-flux irradiation.
Calculation of Irradiation Yield
The following traditional equation is used to project the irradiation yields:
Or
NL176u → number of target atoms 176Lu,σL176u → neutron capture cross section of 176Lu (cm2),N177Lu → number of radioactive atoms 177Lu formed
λL177u → decay constant of 177Lu (s−1),φ → neutron flux (cm−2 s−1),
Integrating Eq. (2) leads to
Owing to the abnormally high neutron absorption cross section (σ = 2065 barn), target burn-up and consumption of product atoms must be considered when undertaking long-term irradiations.
Here
Equation (4) is generally used, provided the neutron capture cross section in the thermal neutron area is inversely proportional to the neutron velocity (the 1/Vn law). However, owing to the resonance in the cross section of 176Lu (Table 2) in the thermal neutron energy range [47], the neutron capture cross section of 176Lu does not follow the 1/v law. Under this premise, using the simplified Westcott convention is an effective proposition for the calculation of the reaction rate of 176Lu(n,γ)177Lu in which an additional correction of the activation rate as a function of the thermal neutron flux temperature is necessary to account for the non-abeyance of the 1/Vn law. This is expressed by the factor k [48–50]. Following the simplified Westcott convention and taking into the account the target burn-up of 176Lu as well as 177Lu atoms during irradiation, the accumulation of 177Lu can therefore be expressed by a differential equation:
The solution of Eq. (5) gives rise to
Here,
Gth and Gr are, respectively, the thermal and epithermal neutron self-shielding factors (both can be set equal to 1 if diluted samples are irradiated), g(Tn) is the Westcott factor [51] ,
The activity produced ‘A’ (in Bq) at the end of irradiation can therefore be calculated using the formula:
The ‘k’ value is the so-called ‘k-factor,’ and the value of ‘k’ is reported to be between 1.5–2.5 [49]. The expression of the yield of 177Lu (Eq. 7) is based on the assumption that the neutron flux is highly thermalized and there is practically no contribution of epithermal neutrons toward 177Lu formation. Based on Eq. 7, the variation of yield of 177Lu per mg of target irradiated with the duration of irradiation is shown in Fig. 3 when an enriched (82 % in 176Lu) target is irradiated at a thermal neutron flux of 1.2 × 1014 n.cm-2.s-1. Figure 3 shows that the yield of 177Lu passes through a maximum and then decreases as the time of irradiation increases. With increasing the ‘k’ value, the yield of 177Lu during neutron irradiation increases.
Variation of the specific activity of 177Lu, theoretically calculated using k = 1.5, 2.0 and 2.5, with the duration of irradiation when the enriched (82 % in 176Lu) target is irradiated at a thermal neutron flux of 1.2 × 1014 n.cm-2.s-1r
The time of irradiation at which the maximum activity of 177Lu is achieved is expressed as [50]
It is evident from the equation [43] that by increasing the neutron flux and/or k-factor, a shorter period of irradiation is required to achieve the optimum yield of 177Lu. It is worthwhile to point out that an irradiation period equal to tmax does not provide the maximum specific activity owing to the transformation of the target material in the nuclear reaction. As reported by Zhernosekov et al. [50] , the actual specific activity of 177Lu is different from the value obtained by dividing the production of the yield of 177Lu by the mass of the target irradiated, since the actual mass of lutetium present in the system post irradiation is different from the initial mass of the target irradiated owing to target burn-up. During irradiation, 177Lu absorbs neutron and leads to the formation of 177/178Hf, which results in the accumulation of the 177/178Hf carrier in the target system. While the presence of 177/178Hf(IV) has no consequences in the efficiency of 177Lu(III)-labeling reactions [53], accumulation of hafnium atoms decreases the specific activity.
Using the burn-up correction, the actual specific activity S (Bq/mol) of 177Lu can be expressed as:
Figure 4 compares the variation of the burn-up corrected specific activity of 177Lu calculated using Eq. 9 with these calculated values without taking target burn-up into consideration. It is evident from the estimates that the period of irradiation at which the maximum yield of 177Lu is achieved does not provide the highest available specific activity. Theoretical calculation shows that the available specific activity (burn-up corrected) of 177Lu passes through a maxima at ~21 days of irradiation when the enriched Lu target (82 % 176Lu) is irradiated at a thermal neutron flux of 1.2 × 1014 n.cm-2.s-1. This duration is significantly higher than the theoretically calculated ‘tmax’ of 177Lu yield, which is ~14 days at the same irradiation condition. Irradiation longer than the ‘tmax’ leads to some loss of activity, but also to an increased 177Lu/176Lu ratio and hence increased specific activity due to burn-up of 176Lu. This theoretical analysis justifies the 21-day irradiation cycle used for 177Lu production in the Dhruva reactor in India [54, 55]. The Indian experience has demonstrated that the theoretically calculated value of the actual or available specific activity of 177Lu after 21 days of continuous irradiation of an enriched Lu target (82 % 176Lu) at a thermal neutron flux of 1.2 × 1014 n.cm-2.s-1 (1,142 GBq/mg, using k = 2.5) is close to the practically obtained value (1,108 ± 24 GBq/mg) [54, 55] .
Comparison of variation of calculated 177Lu specific activity taking into account the burn-up correction with that calculated without burn-up correction (both considering k =2)
While the calculation of 177Lu yield based on the simplified Westcott convention is precise enough, the utility of this computation requires accurate knowledge of the neutron flux parameters of the reactor. The accuracy of the 177Lu irradiation yield calculation strongly depends on the stability of the neutron flux parameters during the target irradiation period. The relatively small variations in the calculated and actual 177Lu-specific activities are mostly due to the variation of the neutron flux levels due to the power level of the reactor operation. It is not practicable to normalize the neutron flux level in the multipurpose research reactor.
Therefore, the maximum obtainable specific activity that could be achieved through the direct production route is about 70 % of the theoretical value; this is only possible for irradiations conducted in high neutron-flux nuclear reactors, which are available in a limited number of countries. It has been reported that it is possible to achieve specific activities of 1,850−2,405 GBq/mg (50−65 Ci/mg) by irradiation in higher flux reactors such as the HIFR reactor at Oak Ridge National Laboratory [56, 57]. Lutetium-177 with specific activity values of >740−1,110 GBq (20–30 Ci)/mg could be produced using an enriched 176Lu target up to approximately 60–80 % in medium flux reactors [54, 55] . The SA values are adequate for all established applications of 177Lu for radionuclide therapy.
While using lutetium oxide enriched in 176Lu up to approximately 60–80 % constitutes a successful paradigm for producing 177Lu of specific activities >740 GBq (20 Ci)/mg amenable to radionuclide therapy, the coproduction of 177mLu with a half-life of 160.1 days owing to the 176Lu(n,γ)177mLu (σ = 2 barn) nuclear reaction has emerged as one factor that may be an impediment restricting its utility in some countries. The 177mLu content in the final product depends not only on the irradiation time, but also on the time elapsed after the end of the irradiation (EOI). Under this premise, it is pertinent to note that owing to the long half-life and low neutron absorption cross section, the activity levels of 177mLu formed will be low but still be of possible concern. Whereas the 177mLu waste issue must be locally resolved, estimates have clearly shown that the resulting radiation dose increase from the presence of 177mLu is insignificant at clinically significant dose levels, at least for PRRT [53]. Under the optimized production conditions, the reported values for the 177mLu/177Lu ratio vary between 0.01 %–0.02 % at EOB [57].
The presence of 177mLu may raise the following concerns:
Radiation dose: As hospitals are using their 177Lu for the preparation of radiopharmaceuticals up to 1 week after EOB, the 177mLu/177Lu ratio would likely be doubled. A usual therapeutic dose of 177Lu ranges between 7 and 9 GBq. When the 177mLu/177Lu ratio is 0.02 %, this means that a dose includes approximately 1.4–1.8 MBq 177mLu.
Laboratory waste: During the radiolabeling process and treatment, the loss of radioactivity is typically 2 to 5 % of 177Lu activity, which corresponds to levels of 28–90 kBq 177mLu. In view of the permissible release limit for 177mLu waste (10 Bq/g), all laboratory radioactive waste is required to be collected separately and shipped to a radioactive waste management facility where it is allowed to decay. With a half life of 160.1 days, a considerable amount of time is required to decay 177mLu.
Waste water: A patient excretes approximately 80 % of the administered dose (1.45 MBq 177mLu) after administration of 177Lu-labeled octreotide through the urine. The patient-excreted activity in urine and feces must be stored in waste water where there is a considerable chance of accumulation of 177mLu in the radioactive waste water holding tanks. According to the European radiation safety regulation, the maximum permissible radioactive concentration of 177mLu in the municipal sewage is 50 kBq/m3 [58]. This means that radioactive waste water from the holding tanks needs to be diluted significantly before discharging into the municipal sewage line. The presence of 177mLu might exceed the activity limits alone or with other nuclides (sum activity) in the radioactive waste water holding tanks and must be evaluated in each case.
While the radiation dose to patients from 177mLu (0.01 %–0.02 %) is of little consequence [53], the problem of safe handling and disposal of the residual quantities of 177mLu by the hospital user may emerge as a major roadblock, which can hardly be circumvented, in principal, through the storage of the radioactive wastes that is customary in hospitals. Despite the above-mentioned drawbacks, however, 177Lu obtained from the (n,γ) route is preferred by many hospitals owing to the cost-effective availability of acceptable quality and quantities on demand. This can be seen as the window of opportunity to lay the basis for realizing the widespread radiopharmeutical use of 177Lu.
Indirect Production Route [176Yb(n,γ)177Yb β−−→ 177Lu]
The indirect production route offers the following advantages:
The highest >2.96 TBq (80 Ci)/mg vs. theoretical 4.07 TBq(110 Ci)/mg specific activity of 177Lu is attainable by this production route.
Offers the potential to provide 177Lu of the highest possible radionuclide purity.
The presence of long-lived radioactive impurities (e.g., 177mLu, <10–5 %) is precluded (below the detection limit) and therefore associated with minimum radiation protection and waste disposal issues.
Specific activity is independent of neutron flux.
Offers satisfactory radiolabeling performance.
The 177Lu obtained by this method has a longer shelf-life (up to 2 weeks) owing to no appreciable decrease in specific activity.
However, this production route also has the following shortcomings, which may be expected to obstruct the path toward widescale utility.
Low production yields due to the poor 176Yb thermal neutron reaction cross section (2.5 barn) as compared to the 2090 barn for the “direct” production from 176Lu.
The effective separation of micro amounts of 177Lu from macro amounts of the irradiated Yb target is not only challenging, but also requires an elaborate radiochemical separation as well as purification procedure.
Generates significant amounts of radioactive waste.
By far, this method of production emerges as the most expensive option to obtain 177Lu of requisite purity.
Not only requires an enriched 176Yb target but also its recovery and recycling.
Despite the above-mentioned drawbacks, there are tremendous prospects associated with the use of NCA 177Lu in TRT; hence, this production route is being aggressively pursued by several institutions. The inherent success of this production route resides in the development of an effective strategy for the efficient separation of pure 177Lu from bulky masses of the neutron irradiated Yb target since Yb follows an identical coordination chemistry with the chelating agents used for the preparation of Lu-based radiopharmaceuticals as well as successful recovery of the Yb target for recycling.
Selection of the Target
As described in Table 2, natural ytterbium consists of a mixture of seven stable isotopes, including 168Yb, 170Yb, 171Yb, 172Yb, 173Yb, 174Yb and 176Yb, among which 174Yb is the most abundant.
Using natural Yb is a major deterrent because of the following:
Neutron irradiation of natural Yb will lead to the co-production of 169Yb (T½ = 32.026 d) and 175Yb (T½ =4.185 d). The presence of these contaminants will not only complicate the irradiated target handling owing to augmentation of the radiation dose, but also involves higher shielding requirements. The radiation dose to the chemical reagents used for sequestering 177Lu will be significantly higher and may lead to radiation degradation.
Generates significant amounts of radioactive waste.
While the cooling of the irradiated target is an effective measure to reduce the contribution of 169Yb and 175Yb, this will reduce the yield of 177Lu owing to radioactive decay.
Decay of 175Yb via β- emission to 175Lu leads to the accumulation of stable lutetium, which will decrease the specific activity of the separated 177Lu.
In view of these considerations, assessing the potential of enriched 176Yb (up to ~97 %) is not only an interesting prospect, but may also be viewed as a necessary one. Owing to the low target burn-up of enriched 176Yb during production, development of a process for the recovery of the unused enriched target is one of the key factors that would be expected to ultimately contribute to the economic success of 177Lu production by this indirect route.
Chemical Form of the Target
While the use of a metallic target is successful for neutron irradiation, using ytterbium metal as a target is precluded as it readily oxidizes in air and under oxygen. Furthermore, the implicit need to use concentrated acid to dissolve irradiated Yb metal continues to thwart efforts toward its utilization as a target for neutron irradiation. In this context, the use of Yb2O3 is the only practical choice since it not only possesses sufficient chemical and thermal stability under reactor irradiation, but also allows easy post-irradiation processing by simple target dissolution in dilute acid.
Irradiation Yields
In this case, the net production rate of nuclide 177Lu is given by
Assuming that the number of target atoms, NYb, remains constant (no considerable
target burn-up) and NYb = NLu = 0 at t = 0 (start of irradiation), integration of Eq. (1) gives rise to
Here NYb is the initial number of 176Yb atoms, σYb is the cross section of the 176Yb(n,γ)177Yb reaction, ϕ is the neutron flux of the irradiation source, t is the irradiation time, td is the decay time after irradiation, and λYb and λLu are the decay constants of 177Yb and 177Lu, respectively. Figure 5 compares the calculated yields of production of 177Lu by the indirect route at different neutron flux values and different irradiation times.
Chemical Separation of 177Lu from Neutron-Irradiated 176Yb
While the indirect 177Lu production method resides at the interface between many disciplines, the inherent determinant for the success of this production route lies in the selection of an appropriate Yb/Lu radiochemical separation. The isolation and purification of 177Lu from the neutron-irradiated target have been subjects of considerable interest. The technical issues associated with the separation of microscopic levels of 177Lu from the macroscopic levels of the 176Yb target represent a challenging task. With a view to realizing the objective, it is imperative to evaluate the differences in chemical and physical characteristics of the Yb and Lu elements that could be used to obtain 177Lu of the requisite purity and yield.
As shown in Table 3, the chemical properties of Yb and Lu are very similar. As a result of the similar characteristics of the group elements, the stability constants of metal ions with a particular ligand show only slight differences. However, such ligands could provide the potential for the separation of these two ions using either ion-exchange chromatography or a solvent extraction technique. Careful scrutiny of Table 3 reveals that the differences are only observed with the existence of a relatively stable oxidation state +2 for Yb and the high solubility of metallic Yb in mercury.
Table 3
Chemical and physical characteristics of Lu and Yb
| Properties | Yb | Lu |
|---|---|---|
| Electronic configuration | [Xe]4f 146s 2 | [Xe]4f 145d 16s 2 |
| Ionic radius of Ln3+ [pm] | 86.8 | 86.1 |
| Ionic radius of Ln2+ [pm] | 114 | - |
| E0(Ln3+/Ln) | -2.267 | -2.255 |
| E0(Ln3+/Ln2+) | 1.05 | - |
| Solubility in Hg | High | Low |
Due to the fully filled 4f subshell, unlike lutetium, the oxidation state Yb+2 is relatively stable. The properties of Yb2+ are very similar to group 2 metal cations such as Ca2+ and Sr2+. Therefore, Yb2+ forms an insoluble sulfate, whereas Lu3+ does not. This property has been exploited for separation of the Yb+3 and Lu+3 ions. The principal shortcoming of this method is that the separation is not clean and requires multiple steps in order to achieve satisfactory decontamination.
Ion exchange separation is of course also possible using cation exchangers and elution by complexing agents. In this case the difference in the stability constant of Yb and Lu with the complexing agents is exploited to realize separation. The order of elution of Yb and Lu depends on the values of stability constants of the formed complexes, and the one that forms a strong complex is initially eluted.
In addition, the selective reduction of Yb can be judiciously exploited to achieve separation from Lu. Alkali metal amalgam, which is one of the strongest reducing agents, can be used for the reduction of Yb3+ to Yb2+ as well as Yb2+ to the free element, which can then enter the mercury phase owing to its ability to form amalgam with Hg.
Technological realization of Yb/Lu separation strategies poses several challenges and requires a thorough assessment to evaluate their prospects. The following are some key features that must be taken in to consideration when selecting a separation process.
The chemical separation process chosen must be effective for the separation of micro amounts of 177Lu from macro amounts of Yb.
Separation must be performed rapidly not only to minimize decay losses of 177Lu, but also to reduce the radiation dose to reagents to circumvent radiation degradation.
The separation method selected should be proficient to provide 177Lu with the highest possible decontamination factor from ytterbium.
The process must ensure consistency, reproducibility and high product yield (ideally > 85 %) on a continual basis.
The separation process should be capable of providing 177Lu in a suitable chemical form (ionic form) amenable to radiolabeling with a broad class of carrier molecules.
To undertake production of 177Lu on a weekly basis annually makes it essential to have a very high degree of robustness of the separation process. Nevertheless, the process should be simple, safe and insensitive to subtle variation in operating parameters.
Amenability to safe operation in a remotely operated shielded facility with negligible operational constraints.
Flexibility to scale up or down to its level of operation in response to requirements.
Generates a minimum quantity of radioactive waste.
Offers the possibility of recovering the ytterbium for target recycling.
Taking into consideration the above-mentioned criteria, a great deal of effort has been expended upon the development of a number of Yb/Lu separation strategies. Essentially every conceivable separation approach has been profusely exploited. Methods ranging from ion exchange chromatography to electrochemical separation strategies have been employed. Brief overviews of these approaches are elaborated in the following sections.
Ion-Exchange Chromatography
Among the techniques used in radiochemical separation, ion-exchange chromatography has been generally proved to be a widely utilized, reliable, and straightforward way to separate radionuclides of interest for myriad applications. While the ion-exchange chromatography technique is appealing in terms of operation simplicity and amenability to a remotely operated facility, a straightforword separation of Yb and Lu is a difficult task owing to their striking similarities in chemical properties. Following a somewhat different path is required in which both Yb and Lu can be adsorbed on a cation exchanger and elution by an appropriated complexing agent. In this premise, two equilibria are required to be considered, i.e., the equilibrium between the complexing agent and the ion exchanger and the equilibrium between the Yb and Lu and the complexing agent. The difference in stability constants for the Yb and Lu with complexing agents forms the genesis of separation. The order of elution of Yb and Lu as well the resolution of the elution band depends on the stability constant values of the formed complexes. Since the smaller ions show a greater preference for complexation, Luaq3+ is the first to emerge from the column, followed by Yb. While the well-characterized α-hydroxyisobutyrate (α-HIBA) complexant as an eluting agent is useful for the separation of Lu from Yb, a Lu/Yb separation factor (α) of only 1.55 [59, 60] has been a major limitation. Owing to the low separation factor, the lutetium fraction contains significant levels of ytterbium because of the “peak tailing.” Moreover, the α-HIBA complex of 177Lu is not optimally suited for the routine synthesis of 177Lu-labeled radiopharmaceuticals. In light of the explicit need to use 177Lu for the preparation of radiopharmaceuticals, α-HIBA must be decomposed and removed prior to labeling because of its high stability constant. The transfer of 177Lu out of the thermodynamically very stable 177Lu-α-HIBA species prior to labeling constitutes a necessity as its presence not only leads to poor labeling yield, but also requires post-labeling purification. In an attempt to circumvent this drawback, one of the methods used for the removal of α-HIBA is the adsorption on a cation exchanger followed by elution with ~9 M HCl [61]. Use of ethylene-diamine-tetra-acetate or 1,2-diamino-cyclohexanetetraacetate (α = 1.7) in lieu of α-HIBA met with limited success because of solubility problems and the requirement of additional steps to obtain 177Lu of desired purity amenable for the preparation of radiopharmaceuticals [62]. Despite these inherent shortcomings, interestingly and surprisingly enough, the enthusiasm for using the ion-exchange chromatography technique has resulted in some investigators evaluating this pathway.
Balasubramanian reported [63] the separation of 177Lu from 10.35 mg of neutron-irradiated ytterbium using Dowex 50 W × 8 (200–400 mesh), a cation exchanger in Zn2+ form. 177Lu was eluted using 0.04 M α-hydroxyisobutyric acid at pH 4.6 ± 2 at 26 ± 1 °C in about 4 h. Lutetium-177 was separated in 70 % yield and with a radionuclidic purity greater than 99 %. While the reported method was successful in isolating 177Lu, more than 30 % of 177Lu contaminated with ytterbium was sacrificed. Lutetium-177 obtained from this method has low specific volume and contains the barrier-ion Zn2+. The presence of Zn2+ in the eluate is a major obstacle in the complexation chemistry of 177Lu and therefore necessitates purification as well as concentration prior to labeling.
On a similar theme, Hashimoto et al. reported the utility of reversed-phase ion-pair chromatography using a Resolve C18 column in which 177Lu was eluted with a mixture of 0.25 M α-HIBA as a complexing agent and 0.1 M 1-octanesulfonate as an ion-pairing agent [64]. In this procedure, 5 mg of the neutron-irradiated Yb2O3 target was used, and the process was effective to provide radiochemically pure 177Lu with 84 % yield. Although this method was productive with small amounts of Yb2O3 target (0.01-1 mg), the separation efficiency deteriorated when higher amounts of the Yb2O3 target were used, resulting contamination from distortion of the ytterbium peak tailing into the lutetium peak.
Solvent Extraction
Liquid-liquid extraction is one of the most promising techniques often used for radiochemical separation. Significant practical experience has accumulated over the years in using this technique in a highly radioactive environment and on an industrial scale. Although use of the liquid-liquid extraction method based on the different extractability of Lu and Yb acidic organ phosphorus extractants holds promise, the requirement of a multistage process, which is essential to achieve the necessary decontamination of Yb from Lu owing to the low Lu/Yb separation factor (α), constitutes the major impediment that probably limits its wide-scale utility. The liquid cation exchanger, di-(2-ethylhexyl)phosphoric acid (HDEHP), has effectively been utilized as an extractant for the isolation of 177Lu in proton-activated ytterbium [65]. In this method, 0.2 g of proton-irradiated nat Yb2O3 target was dissolved in 1 M HCl, and the 177Lu formed was extracted in the organic phase containing 1 % HDEHP in cyclohexane. It is apparent that liquid-liquid extraction for 177Lu separation is still in its infancy and presently represents a potential separation technique, but much additional effort is required in order to realize its potential.
Supported Liquid Membrane Extraction
The supported liquid membrane (SLM) method for Lu/Yb separation has its roots in the liquid-liquid extraction method in which a Lu-selective organic extractant is impregnated on an inert semipermeable membrane, and separation of Lu is achieved by its selective transport through the pores of the impregnated membrane. In order to tap the potential of SLM for the radiochemical separation of 177Lu from Yb, HDEHP in hexane was impregnated on a membrane consisting of two blocks (one made of PVDF and the other of PTFE) with identical channels of dimensions. The membrane thickness was 200 μm, and its nominal pore size was 0.2 μm. The donor side of the membrane contained 0.2 mol dm−3 of ammonium acetate buffer at pH 5–5.5 in which the neutron-irradiated Yb target solution was added and the acceptor side contained 2 mol dm−3 HCl in which 177Lu was collected [66]. Despite promising results, this separation procedure has never been extended for the separation of 177Lu from neutron-irradiated Yb. The scale of 177Lu separation possible by this route will be limited but could still be of interest and utility in meeting local needs. Continuing research in this separation methodology can be expected in the near future.
Extraction Chromatography
An alternative to liquid-liquid extraction is the possibility to incorporate an extractant or a solution of an extractant into an inert substrate that can be used as a support in a column chromatographic technique. The most striking feature of the extraction chromatography (EXC) technique is that it combines the selectivity of liquid-liquid extraction with the ease of operation and rapidity of a column-based separation system. It is critical, however, that an appropriate extractant needs to be chosen that offers a satisfactory Lu/Yb separation factor (α).
The EXC technique has been explored by Knapp et al., leading to the development of a one-step extraction chromatography separation process [56, 57, 67] and making use of the commercially available LN Resin, which comprises di(2-ethyl-hexyl) ortho-phosphoric acid (HDEHP), commercially available from Eichrom Technologies, Inc. The reported method was found to be effective for the quantitative separation of 177Lu from 10 mg of nonradioactive Yb carrier using HCl of different concentrations for sequential elution of 170Tm 176Yb and 177Lu. The elution sequence consisted of an initial elution with 2 M HCl (fraction 1) followed by increasing the acid concentration to 3 M HCl (fraction 2) and then 6 M HCl (fraction 2). The first peak (fraction 1) in the chromatogram contained 170Tm formed by neutron activation of a stable 169Tm impurity in the enriched 176Yb target material. The ytterbium peak (fraction 2) then appeared and finally the 177Lu was eluted with 6 M HCl. The specific activity of 177Lu obtained by this method was estimated to be 3.7 TBq (100 Ci)/mg (i.e., 91 % of the 110 Ci/mg theoretical value).
The aforementioned EXC technique was further exploited by Horwitz et al. and culminated in a conceptual flow sheet that was found to be successful for the separation of NCA 177Lu from a 300-mg irradiated ytterbium target [68]. The process is essentially based on the use of two different EXC resins, namely a resin containing HEH[EHP] (LN2) and a resin containing tetraoctyl diglycolamide (DGA) sorbed onto Amberchrom® CG-71. The whole separation process can be broadly divided into three steps: (1) the front-end target removal step, (2) primary separation step and (3) secondary separation step. While the goals of each separation step differ, it basically consists of separation of Yb and Lu using the LN2 resin followed by the concentration and acid adjustment of the Lu-rich eluate using Amberchrom® CG-71 resin. The use of the diglycolamide EXC material to purify the Lu-rich eluate is the novelty of this technique. Using Amberchrom® CG-71 resin seemed attractive as it precludes lengthy evaporations and acidity adjustments between successive LN2 resin column runs and at the same time is effective in removing adventitious metal ion impurities from the 177Lu fraction. It is worth mentioning that during the purification of the 177Lu fraction by LN2 resin, metal ions such as Zr4+, and Hf3+ (177Hf is the daughter of 177Lu) are strongly retained and therefore free 177Lu from metallic impurities. With a view to eliminating all traces of nitrate ions, a small anion-exchange column in the final step of the secondary separation step has been added. All the 176Yb fractions of the target removal step, primary separation step and secondary separation step were pooled together and could be used for recycling in successive neutron irradiations.
A notable feature of this method is thus the recovery of the isotopically enriched 176Yb target material. The individual decontamination factors for the front-end target removal system, primary separation system and secondary separation system are 101, 102 and 103, respectively. The overall recovery of 177Lu was reported to be 73 %. The total processing time employing the three steps was reported to be 4 h. The simplified flow sheet of the front-end target removal step, primary separation step and secondary separation step are depicted in Figs. 6, ,77 and and8,8, respectively. This method is attractive owing to the commercial availability of LN2 and Amberchrom® CG-71 resin, adaptation of the user-friendly EXC process, shorter processing time, satisfactory 177Lu yield and amenability to routine remote operation as well as automation, and it offers the potential to recover the enriched 176Yb target for recycling. The prospects of adopting such a scheme appear promising for the routine production of NCA 177Lu .

In another independent study, a multicolumn solid-phase extraction (SPE) chromatography technique using di-(2-ethylhexyl)orthophosphoric acid (HDEHP)-impregnated, OASIS-HLB sorbent-based SPE resins (OASIS-HDEHP) was used [69, 70] for the separation of 177Lu from a 50-mg Yb target irradiated in a nuclear reactor with medium neutron flux (ϕ = 5 · 1013 n · cm−2 · s−1). The reported technique exploited the selectivity of OASIS-HDEHP resin for Lu in different concentrations of HCl solution for the consecutive loading-eluting cycles performed on different columns. The method was successful for the isolation of several hundred mCi of NCA 177Lu using a 50-mg Yb target irradiated in a medium neutron flux nuclear reactor (ϕ = 5.1013 n/cm2/sec). The overall separation could be carried out in 5-6 h.
Electrochemical Method
As the name suggests, the electrochemical separation strategy exploits the difference between the standard reduction potentials of two radionuclides in an electrolytic medium to selectively deposit the radionuclide of interest under the influence of the controlled applied potential. The inherent advantages of electrochemical separation processes have been elaborately discussed in recent reviews [71, 72].
While the selective deposition of the radionuclide of interest from ionic state to metallic state under the influence of the controlled applied potential is a successful paradigm, applicability of this strategy for Lu/Yb separation is precluded owing to the deeply negative reduction potentials of lanthanides (more negative than hydrogen discharge) and difficulty in controlling their electrolytic deposition onto the solid cathode. In light of the perceived need to realize the potential for Lu/Yb separation following an electrochemical strategy, a somewhat different path is required. This alternate electrochemical path basically consists of selective reduction of Yb3+ to Yb2+ and its preferential transfer onto a mercury cathode exploiting the ability of Yb2+ to form amalgams with Hg.
This strategy seems attractive for the following reasons:
An examination of the redox potentials of the Yb and Lu indicates the possibility of Yb forming the bivalent state, whereas in the case of Lu, a stable bivalent state is unknown.
While Yb2+ is known to form an amalgam, Lu3+ cannot [73–76]. Therefore, Lu is difficult to deposit on the Hg cathode from aqueous electrolytes.
Offers the possibility of electrolytic reduction of Yb3+ to Yb2+ in a mildly acidic solution owing to its high hydrogen over-voltage. Such an attribute ensures no reoxidation of Yb2+ and offers easy handling and deposition of Yb onto Hg.
The electrochemical separation method is essentially based on the formation of the Yb amalgam by electrolysis into a mercury cathode or extraction into an amalgam aimed at its removal from the Yb-Lu mixtures. With a view to removing Yb, the potential of using Hg is enticing because of its high density, the insolubility of mercury in aqueous medium and the absence of adsorptive effects. In the quest for innovative approaches to separate Yb from other lanthanides, Marsh successfully exploited the electrochemical pathway using a mercury cathode [73–76], which represents one of the very early electrochemical separation strategies at a time when the utility of the electrochemical technique in separation science had not yet been established. This elegant separation technique was later effectively harnessed by McCoy, which paved the way for the first laboratory-scale separation of Lu from Yb [77] and showed the extraction was quite specific for Yb. Extending this theme, Onstott [78, 79] reexamined Yb reduction using a series of alkali metal salts and employed lithium citrate in place of potassium citrate.
These three successful preparative-scale separations of Lu from Yb using mercury cathodes have been reported in the literature and are discussed below.
Cementation Process
In order to tap the potential of the electrochemical method, Lebedev et al. reported a method [80] that essentially consists of dissolution of irradiated Yb2O3 in hydrochloric acid, addition of sodium acetate to form sodium amalgam and extraction of Yb by sodium amalgam from Cl–/CH3COO– electrolytes into mercury. A series of four successive cementation steps each was performed in order to achieve a satisfactory decontamination factor. With a view to achieving the desired purity, the 177Lu precipitate containing trace amounts of ytterbium was dissolved in acid and adsorbed on a cation-exchange column from which 177Lu was selectively eluted using α-HIBA. In light of the explicit need to remove α-HIBA, the eluted 177Lu solution was then adsorbed on a cation-exchange column wherein both Lu and Yb were adsorbed and 177Lu was eluted with 9 M HCl. The recovery yield of 177Lu in this process was 75 %, and decontamination factor from ytterbium was found to be >106. While the reported method is appealing in terms of recovery yield of 177Lu and product quality, the requirement of a time-consuming, complicated process involving multiple cementation cycles together with the elaborate purification steps emerged as the major impediment that would be expected to restrict its wide-scale applicability. The logistics are expected to be unfavorable to carry out such a complicated process on a very regular basis.
In order to mitigate the limitation of this method, Bilewicz et al. [81] developed a method based on the reduction of Yb(III) to Yb(II) with sodium amalgam followed by removal of Yb by selective precipitation as the sulfate. The principal shortcoming of this precipitation method is that the separation is not clean and requires an additional ion exchange purification step to achieve the desired purity amenable for clinical use. While the reported method obviously holds promise, the processing is quite complex because of several factors influencing its performance and requires a purification step to achieve satisfactory purity. This separation strategy is not only user-unfriendly, but also could lead to varying consistencies of the purity as well as yield.
Electro-Amalgamation Process
The electro-amalgamation approach developed by Chakravarty et al. [82] is based on the electrolytic reduction of Yb3+ to Yb2+ in lithium citrate medium followed by formation of Yb amalgam by electrolysis and extraction of Yb from the mercury cathode. A schematic diagram of the electrochemical setup used in this procedure is depicted in Fig. 9. In the two-cycle electrolysis, the first step is is the pre-elimination of the bulk of the Yb target mass, and the second step is the further purification of 177Lu. This process provides NCA 177Lu with acceptable purity and satisfactory separation yield (>90 %) within 3-4 h. The flow chart of this electro-amalgamation process is shown in Fig. 10. This strategy thus far has been confined to laboratory-scale investigations but could still be of interest and utility if adequate technological attention is imparted. The prospects for adopting such a scheme appear promising in the foreseeable future.
Accelerator-Based Production of 177Lu
Accelerator technologies could be used to produce small quantities of 177Lu, and although a number of routes can be explored that would be useful in basic research, these are not expected to really serve as the basis to undertake large-scale cost-effective production because of the extremely low cross sections of the reaction routes envisaged.
With a view to realizing the accelerator production of 177Lu, a number of studies concerning activation cross sections of the deuteron-induced nuclear reactions as well as excitation functions of the natYb(d,xn)177,173,172mg,171mg,170,169Lu reactions have been reported, and the following are of interest. Hermanne et al. studied the cross sections of deuteron-induced reactions on Yb targets and measured the cross sections between 3 and 20 MeV for Yb(d,xn)170Lu/171Lu/172Lu/173Lu/174Lu/177Lu, and Yb(d,xnp)169Yb/175Yb [83]. Manenti et al. measured the activation cross sections of Yb(d,xn)169Lu/170Lu/171Lu/172Lu/173Lu/174Lu/176Lu/177Lu,Yb(d,xnp)169Yb/175Yb/177Yb reactions up to 18.18 MeV [84].
Tárkányi et al. performed a systematic study of the activation cross sections of deuteron-induced nuclear reactions and excitation functions of the natYb(d,xn) 177,173,172mg,171mg,170,169Lu,natYb(d,x)175,169Yb and natYb(d,x)173,172,168,167,165Tm reactions up to 40 MeV. Some of these reactions were evaluated for the first time [85]. Although promising, substantial R&D and large resources are required for the technological development and assessment owing to the challenges associated with target preparation as well as the sustained operation of accelerators on a reliable and continuous basis.
Quality Control of 177Lu
Having reviewed in detail the direct and indirect strategies for reactor production and the various processing technologies used to obtain 177Lu, the quality evaluation of 177Lu is the next important issue with regard to providing this radioisotope for clinical use. Because of the requirements imposed by pharmaceutical legislation to ensure safety and efficacy, despite these encouraging prospects and the favorable results of the various production and processing methodologies, quality evaluation of 177Lu is, of course, a prerequisite before preparation of radiopharmaceuticals in the daily nuclear medicine routine.
Radionuclidic Purity
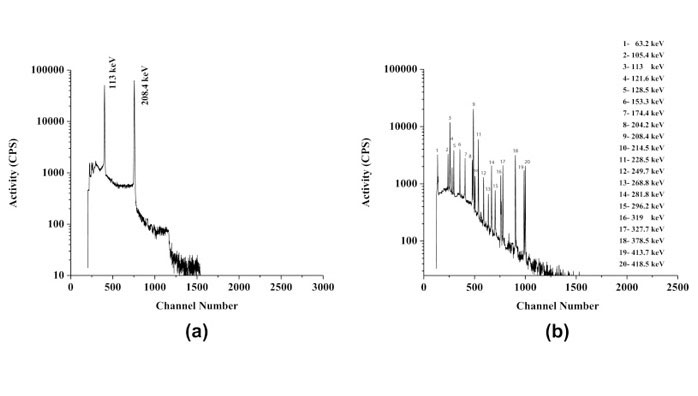
Radionuclidic purity is defined as the ratio, expressed as a percentage, of the radioactivity of 177Lu to the total radioactivity content of the sample. Gamma spectroscopy using a high-purity germanium (HPGe) detector in conjunction with a multichannel analyzer (MCA) is used for routine determination of the radionuclidic purity of 177Lu. To be able to quantify the 177Lu and the possible impurities, the detector system must be properly calibrated for both energy and efficiency using either a series of standardized sources, each containing a single radionuclide, or a single calibrated source containing a radionuclide having several gamma photon of different energies (e.g., 152Eu) obtained from a National Metrology Institute (NMI) or commercial laboratories that can demonstrate measurement traceability to an NMI. Because of the requirement to maintain dead time and pile-up at acceptable levels (dead time <10 %) during measurement, it is mandatory to dilute the 177Lu sample appropriately. While gamma spectrometry constitutes a successful example of the determination of the radionuclidic purity of 177Lu, direct spectral analysis of 177mLu co-produced with 177Lu and other longer lived contaminants is not possible because of the overwhelming contribution of 177Lu. This difficulty is mitigated by keeping an aliquot of a 177Lu sample, which is allowed to decay for an appropriate time (i.e., ~60 days), and then analyzing it using the gamma spectrometric technique. Gamma photon peaks pertaining to 177mLu and other long-lived radionuclides can then be easily identified based on their characteristic gamma rays. A typical gamma spectrum of 177Lu obtained from the (n,γ) 177Lu production route immediately after radiochemical processing and after 70-day decay is shown in Fig. 11.

A typical gamma spectrum of a 177Lu sample aliquot obtained from the (n,γ) 177Lu production route recorded immediately (a) after radiochemical processing and (b) after 70-day decay
Radiochemical Purity
Radiochemical purity is defined as the ratio, expressed as a percentage, of the radioactivity of 177Lu present as 177LuCl3 to the total radioactivity of 177Lu present in the sample. With a view to determining the radiochemical purity of 177Lu after chemical separation, both paper chromatographies (PC) as well as high-performance liquid chromatography (HPLC) techniques are used. Paper chromatography using Whatman 3MM strips is the method most commonly used to test 177Lu for radiochemical purity. The PC method is simple, fast and inexpensive. A small aliquot (~5 μL) of the test solution can be spotted at 1.5 cm from the bottom of a paper chromatography strip. The strip needs to be eluted using 0.9 % NaCl (w/v) in 0.02 M HCl as the eluting solvent. After elution, the strip can be dried and cut into segments (i.e., typically 1 cm). The radioactivity associated with each segment can be determined by using a well-type NaI(Tl) scintillation counter by keeping the base line at 150 keV and with a window of 100 keV, thereby utilizing the 208-keV gamma photon of 177Lu. A typical paper chromatography pattern of 177Lu3+ is shown in Fig. 12. For HPLC analysis, a typical system utilizing water (A) and acetonitrile (B) mixtures with 0.1 % trifluoroacetic acid is used as the mobile phase. A typical HPLC pattern of 177Lu3+ is illustrated in Fig. 13.
Chemical Purity
In light of the explicit need to perform radiolabeling with 177Lu, the chemical purity is also of paramount importance, especially for receptor-targeted agents. In view of the extremely low concentration of 177Lu, the metal ion impurities even at ppb levels act as pseudocarriers, requiring higher concentrations of the targeting vectors to achieve high radiolabeling yields. Recognizing the ability of competing metal ion impurities, such as Al, Ca, Cu, Fe, Pb and Zn, likely to be present in the 177LuCl3 solution, to form thermodynamically and kinetically stable coordination complexes with the targeting vectors, it is of utmost importance to determine their concentration, which could be effectively achieved by the inductively coupled plasma atomic emission spectrometry (ICP-AES) technique.
Another noteworthy chemical impurity is the hafnium isotope, which is produced through the decay of 177Lu, 177Hf (177Lu
Specific Activity
With a view to using 177Lu for targeted radionuclide therapy, the goal of attaining the highest possible specific activity is crucial. With a view to realizing this objective, the presence of cold Lu should be minimized to the extent possible as it acts as a competitor for labeling positions on targeting vehicles. On this premise, determination of the specific activity of 177Lu prior to radiolabeling was deemed worthy of consideration.
The specific activity of 177Lu can be expressed as:
Here A177Lu is the measured activity of 177Lu at any particular point in time, and m177Lu, m175Lu, mLu are the mass of 177Lu, 175Lu and cold Lu present in the sample.
The activity of 177Lu in a given aliquot is generally measured following gamma spectroscopy using an HPGe detector. The sample can be placed for the appropriate time at a suitable geometry, and the counts acquired under 208 keV after chemical processing can be used for assay of 177Lu. The total concentration of 177Lu, 175Lu and cold Lu in the sample can be determined by the ICP-AES technique. From the determination of the activity and total Lu concentration, the specific activity of 177Lu is computed.
The current worldwide suppliers of good manufacturing practices (GMP) producing 177Lu as a radiochemical are provided in Table 4. In addition to the major producers and suppliers, some countries also produce small quantities of 177Lu for domestic use.
Table 4
Current suppliers of GMP-produced 177Lu as a radiochemical
| Suppliers | Specific activity | Chemical form | Category | Radiochemical concentration |
|---|---|---|---|---|
| Perkin Elmer,USA | ~20 Ci(740 GBq) /mg at production | 177Lu as LuCl3 in ~0.05 M HCl. | CA | ~3 Ci(111GBq)/ml on the day of production |
| ORNL, USA | 50–80 Ci(1.85 - 2.96 TBq)/mg | 177Lu as LuCl3 in 0.1 M HCl | CA | 8 Ci(296 GBq)/ml |
| MURR, USA | 25 Ci(925 GBq)/mg | 177Lu as LuCl3 in 0.05 M HCl | CA | 3 Ci(111 GBq)/ml |
| MDS Nordion, Canada | 45 Ci(1.665 TBq)/mg | 177Lu as LuCl3 in 0.05 M HCl | NCA | ≥200 mCi( 7.4 GBq)/ml |
| ITG, Garching, Germany | 80 Ci(2.96 TBq)/mg | 177Lu as LuCl3 in 0.04 M HCI | NCA | 8 Ci(296 GBq)/ml |
| IDB Holland BV | ~20 Ci(740 GBq)/mg | 177Lu as LuCl3 in 0.05 M HCI | CA | 3 Ci(111 GBq)/ml |
Regulatory Requirements and Automation
Lutetium-177 produced from any of these production routes is considered as an active pharmaceutical ingredient (API) since it is used as a starting material for the preparation of radiopharmaceuticals for human use and production must be regulated. The emphasis on quality is most prominently manifested by the fact that all equipment, instruments and technologies in 177Lu production facility and the associated accessories must meet the preset criteria and the product obtained has to meet strict specifications. Written and approved protocols specifying the critical steps and acceptance criteria must be in place. Confirmation of appropriate regulatory conditions for aseptic processing and its supportive activities is mandatory. Production of 177Lu should be carried out according to GMP, which is becoming mandatory in most countries.
In order to achieve GMP compliance, it is essential to have a full documentation system providing traceability that includes:
A Site Master File
Drug Master Files for the individual batch
Validation Master File
Specifications for materials
Operating procedures
Batch processing records
Training of staff
The US Food and Drug Administration (FDA) approved a set of regulations describing production of radionuclides used as APIs according to cGMP, outlined in the Code of Federal Regulations. In order to address these regulatory demands, radionuclide production is migrating toward the use of automated modules. The advantages of using automated production strategies include:
Assuring reproducibility in production yield as well as consistency in product quality.
Improving the robustness of the production as well as providing on-line documentation of the process, thus improving GMP compliance.
Providing a log of the steps performed during the processing of 177Lu. Electronic record keeping is not only accurate and complete, but also helps in accomplishing regulatory compliance.
Precluding the possibility of cross-contamination.
Ability to handle multiple GBq levels of radioactivity safely, enabling the manufacturer to produce and distribute the required quantities of 177Lu for therapy.
Facilitating regulatory compliance through manufacturer installation qualification/operational, qualification/performance, qualification and scheduled maintenance protocols performed for 177Lu production by trained personnel.
Improving radiation safety through the reduction (or elimination) of manual operations.
Use of automated production strategies represents an appealing vision where significant resources and effort have been expended. While automation holds promise and offers numerous advantages, it presents radiochemists with the challenge of re-configuring the chemical processing steps that require integration of several steps while maintaining full automation. Nonetheless, to be effective in addressing the particular regulatory barriers, automated processing modules must be customized to local legislative, regulatory and institutional conditions for which a comprehensively designed and correctly implemented quality assurance system is of utmost importance.
In addition to meeting pharmaceutical GMP and gross domestic product (GDP) regulations, manufacturers undertaking regular production of 177Lu must be licensed by a Nuclear Regulatory Authority (NRA). In this context, it is mandatory for the manufacturer to demonstrate that its facility used for 177Lu production is adequate to protect health and minimize danger to life and property. Additionally, the manufacturer must be qualified to use radioactive material, establish a radiation protection program as well as the controls and procedures for the management, record keeping, accounting and use of radioactive materials.
Summary
This overview of the existing 177Lu reactor production and processing technologies along with the recent developments reveals two competitive options, each having relative advantages and disadvantages. Production of 177Lu is inextricably linked to the advancements in TRT. A wide range of innovative new targets, lead compounds and new radiolabeled ligands as vectors are emerging far more rapidly than over the past decade. As radionuclide therapy is moving to the forefront of molecular-targeted radionuclide therapy of cancer and other diseases, the demand for 177Lu is evolving.
Although the accelerator-based 177Lu production option holds promise as an innovative approach, current global trends in this production route are demonstrably unsustainable both technically and economically. Completing the technological development as well as establishing the economics of this approach is expected to be years away, and its success will depend on how these challenges are tackled in the years to come. Of the two reactor production options discussed, the prospect of using CA 177Lu produced by the “direct” route is appealing as it is the least intricate way to obtain 177Lu of reasonable specific activity and will suffice for most applications. While the “direct” production route is attractive in terms of simplicity in target processing and cost effectiveness, the burden of 177mLu in the final product is the key factor in determining its usefulness. Owing to the inherent requirement of an elaborate intricate radiochemical processing technology for the isolation of NCA 177Lu, significant expertise, skilled technicians and adequate resources for undertaking regular production, the number of commercial radioisotope suppliers of NCA 177Lu remains finite, and its current production capabilities are still limited.
In recent years, targeted radionuclide therapy has been moving from an exotic treatment modality for a very few patients to a mainstream modality. The future of targeted radionuclide therapy is, of course, difficult to predict, and there will be surprising inventions that, as in the past, may have an unexpected application that will continue to fuel the field. These represent the niche areas where NCA 177Lu will have an advantage over CA 177Lu. While undertaking large-scale 177Lu production, it is essential to assess both options, weigh pros and cons, and select the one based on the technical and economic resources. It is important to note that these two reactor production routes should not be approached as competitive, but instead provide 177Lu for a variety of clinical applications to benefit needy patients.
Lutetium-177 seems destined to find important applications in the personalized therapy of patients using low-abundance gamma photons for diagnosis. This paradigm, when properly enforced, would not only provide a clear understanding of the disease following its detection and progression, but also provide vital clues for making decisions about individualized treatments. Administering suitable 177Lu-labeled radiopharmaceuticals in their required doses and providing personalized treatment planning constitute a major step forward to meet the challenges of personalized medicine. Implementation of this regimen is likely to trigger profound structural changes in the treatment strategy and potentially to create a situation where treatments can be tailored to individual patient-specific diseases. Effective harnessing of such a treatment regimen requires a constant and reliable supply of 177Lu of the required quality in the desired quantities at a reasonable cost. Because of the pace at which the personalized therapy scene is evolving, 177Lu production strategies need a vision for today and tomorrow.
The advances made in large-scale 177Lu production so far are exciting, and there are no apparent barriers to its adoption for large-scale production. With the appropriate selection of a production route, it would be possible to envision a future where the scale and potential of 177Lu production technology can be tailored to institutional needs. The progressive fusion of existing 177Lu production technologies with automation can be consciously nurtured in effective ways to respond to GMP compliance and to surmount regulatory barriers. Potentially the infusion of automation into 177Lu production technology may be hastened by the creation of a positive platform for future growth. Interest in and expansion of 177Lu production and processing technologies as well as the development and clinical introduction of 177Lu-based therapeutic radiopharmaceuticals have passed many milestones, and it is expected that broader use and regulatory approval of 177Lu-agents will move forward rapidly.
Conflict of Interest
Ashutosh Dash, Maroor Raghavan Ambikalmajan Pillai and Furn F. (Russ) Knapp, Jr., declare that they have no conflict of interest.
Informed Consent
The manuscript does not contain clinical studies. There is no identifiable patient information in this manuscript.
The information comes from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4463871/